Contact: +91-9711224068
FAUNA
- Printed Journal
- Indexed Journal
- Refereed Journal
- Peer Reviewed Journal
Impact Factor: RJIF 5.53
e-ISSN : 2347-2677, p-ISSN : 2394-0522

International Journal of Fauna and Biological Studies
2016, Vol. 3 Issue 3, Part A
A new dimension in the dengue epidemiology with special reference to the genetic diversity of the virus: A review
Author(s):
Probal Basu, Sajal Bhattacharya
Abstract:
The severity of dengue isrnoften ascribed to secondary infection with a virus belonging to a serotype rndistinct from that of the primary infection. Severe pathogenicity of DENV might be regulated at the genetic level and mayrnbe associated with unusual mutational and recombination events which are therntwo major reasons behind the extensive genetic diversity of DENV. The possible emergence of undesired genetically novel variant DENV inrnthe near future could create further complexity and subsequent complications inrnthe pathogenicity of the disease. This review article is an attempt tornunderstand the significance of the extensive genetic diversity of the denguernvirus (DENV) in the development of greater magnitude of the viral pathogenicity rnas well as in the severity of recurrent outbreaks in the context of changingrnenvironment and epidemiology. DENV antigens have been detected from mononuclearrncells, lymphocytes, Langerhans cells in the skin, neurons, astrocytes,rnendothelial cells and hepatocytes , heart and skeletal muscle. This alteredrntropism of the dengue virus in humans might indicate the fitness strategy ofrnthe virus in urban areas since human is the only possible source of viremic rnvertebrate. Remarkably, the RNA virusrnhas developed the ability to recombine with host dsDNA genomes. Thernextraordinary abilities of RNA virus like DENV may unlock a new vista in denguernresearch, which encompasses the relevant proposition of a momentousrnplausibility of crucial genetic exchange between DENV (+) ssRNA genome andrndsDNA of the human host and thereby thernpossible emergence of genetically novel DENV variants associated with altered pathogenicity
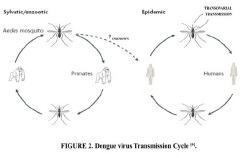
Fig. 1: Dengue virus Transmission cycle
Pages: 29-41 | 2522 Views 541 Downloads

How to cite this article:
Probal Basu, Sajal Bhattacharya. A new dimension in the dengue epidemiology with special reference to the genetic diversity of the virus: A review. Int. J. Fauna Biol. Stud. 2016;3(3):29-41.
Important Publications Links
Related Journal Subscription
Allied Journals
Copyright © 2013 - 2024. All Rights Reserved. International Journal of Fauna and Biological Studies




